ISSN 2410-5708 / e-ISSN 2313-7215
Year 9 | No. 24 | p. 151-161 | February - May 2020
© Copyright (2020). National Autonomous University of Nicaragua, Managua.
This document is under a Creative Commons
Attribution-NonCommercial-NoDerivs 4.0 International licence.
Evaluation of the efficiency of arsenic removal in water with activated carbon from jícaro sabanero (Crescentia alata) and its combination with iron oxides
https://doi.org/10.5377/torreon.v9i24.9724
Submitted on February 13th, 2020 / Accepted on February 20th, 2020
B.A. Kathia Vanessa Rojas Cerda
Laboratory of Metallic Contaminants
Center for Research in Aquatic Resources of Nicaragua (CRAR / UNAN-Managua)
Keywords: Arsenic, activated carbon, iron oxides, removal
Abstract
According to the World Health Organization, arsenic is one of the most dangerous chemicals in the world, due to its potential of causing a wide variety of diseases since diabetes until lung and skin cancers, among others. Therefore, it is important to develop techniques to remove it from drinking water. This study evaluated the arsenic removal efficiency from water using two sorbent media, iron oxides and artisanal activated carbon, in experiments conducted by duplicate for carbon and by triplicate for the sequential treatment iron oxides-carbon. The assays were carried out using activated carbon with two particles size of (2-4 and 0,6 mm) and two contact times (6 and 12 h) to treat samples spiked with three working concentrations (10, 25 and 50 μg As/L). The analysis of the remaining arsenic in water were carried out using the international standard methodology, atomic absorption using the technique of hydrides generation. The highest arsenic removal efficiencies were achieved with the sequential treatment iron oxides-activated carbon: 54, 50 and 58 %, respectively, for the three assayed concentrations. The analysis of variance did not find significant differences between contact time (6 and 12 h), but between particles size of activated carbon. Due to these findings it is recommended to use the shortest contact time (6 h) and the smallest particles size of activated carbon (0.6 mm) for arsenic removal.
1. Introduction
According to the World Health Organization (WHO), Arsenic (As) is one of the most dangerous substances in the world, so it recommends a maximum allowable value for human water consumption of 10 µg As/L (WHO, 1995). This metalloid is found in rocks of volcanic origin such as arsenopyrite (FeAsS) and by the system of geological faults I could leach to the groundwater, being a danger due to its toxicity and carcinogenicity in humans (Esparza, 2006).
In Latin America, arsenic has caused problems in human health since a UN report mentions Argentina, Chile and Mexico as countries in which groundwater contaminated by arsenic has been found. Several studies correlate the intake of inorganic arsenic with lung, skin, bladder and other internal organs (Ferreccio, et al., 2000). Also, there are several non-carcinogenic effects related to arsenic intake at low concentrations, which include: cardiovascular diseases, diabetes, anemia, reproductive, immunological, neurological disorders and complications in pregnancy, such as abortion and premature delivery (Kapaj, Peterson, Liber, & Bhattacharya, 2006).
In Nicaragua, the presence of arsenic levels above the maximum permissible limits in water for human consumption was detected in 1996 in a drilled well in Zapote, Matagalpa, with a concentration of 1 320 µg As/L (UNICEF, 2002). Since that date the arsenic has been detected in other areas of the country, among them are: the Kinuma region in the Municipality of La Libertad, Chontales in which a well with a concentration of 106.7 µg As / L was registered and in Cerro de Agua Mine in the Municipality of Villanueva, Chinandega, a well with a concentration of 12.5 µg As/L was registered (UNICEF, 2004). In addition, in Sébaco located in the department of Matagalpa, arsenic concentrations were detected in 21 wells between the range 10 to 122 µg As/L (Altamirano E, 2005)
There are different methods for arsenic removal such as: precipitation processes including coagulation-filtration, direct coagulation filtration, flocculation and softening (Han, Runnells, & Wickramasinghe, 2002), anion exchange processes (DeMarco, SenGruta, John, & Greenleaf, 2003), membrane filtration (USEPA, 2007) and adsorption processes that include adsorption with iron (III) salts (Mohan & Pittman, 2007). The latter is a process of low cost and high efficiency widely used for the removal of arsenic, in which this metal reacts with the alkalinity of the water to form ferric hydroxides, compounds that form small insoluble conglomerates called “flocs”. Arsenic is linked to floccules formed of ferric hydroxide, which are subsequently removed by sedimentation and / or filtration in granular beds (Amirtharajah & O’Melia, 1990).
On the other hand, activated carbon is widely used in adsorption processes of pollutants (Universidad Politecnica de Sevilla, 2002) so it can remove arsenic flocs formed in the process of complexing with iron oxides. Adsorption is a process whereby the transfer of dissolved molecules in a liquid phase to a solid phase occurs in several stages on the surface of activated carbon (Ngai, Dangol, Murcott, & Shrestha, 2005).
This study was based on the laboratory-scale assessment of the efficiency of hand-made activated carbon based on the jícaro sabanero shell (Crescentia alata) and its combination with iron oxides to remove arsenic from water.
2. Methodology
2.1. Experimental design
Tests for the determination of optimal carbon time and size for the removal of arsenic were performed by subjecting three known concentrations of As (10, 25 and 50 µg As/L) in deionized water prepared from a solution of 1 000 µg As/L diluted from a Titrisol vial, two sizes of activated carbon (2-4 mm and 0.6 mm) and during two contact times (6 h and 12 h). These tests were carried out in duplicate in 355 mL plastic bottles. The lowest concentration tested (10 µg/L) was selected since WHO has regulated this value as the maximum allowable amount of arsenic in water for human consumption and the maximum concentration tested (50 µg As/L) was selected as it has been reported that chronic exposure to similar concentrations is associated with various conditions (Hughes, 2002).
Using the results obtained in the previous tests, the time and size of activated carbon in which the best removal percentages were obtained; the arsenic removal percentages were determined by means of iron oxides and activated carbon. These experiments were carried out with water from the perforated well of the community of El Bonete, to which different volumes of standard arsenic solution were added to obtain 14, 29 and 54 µg As/L; respectively. Then it was proceeded to put in contact with the iron oxides and the activated carbon produced by hand based on jícaro shell (Crescentia alata) of 0.6 mm for 6 h. These tests were performed in triplicate.
The natural water used was collected from the perforated well with a depth of 100 feet, located in the community of El Bonete, municipality of Villanueva in the department of Chinandega, which presented a concentration of 3.6 µg As/L and has the following Basic physical-chemical characteristics: pH = 7.37, Electrical conductivity = 475 µS/cm and Total hardness = 220 mg/L (as CaCO3). The sample was collected and preserved with 2 ml of HNO3 per liter of sample and transferred in a thermos with ice to the Laboratory of Metal Pollutants of CIRA/UNAN for analysis, following the Chain of Custody Protocol of CIRA / UNAN (PROC -CM-02).
2.1.1. Application of activated carbon (2 - 4 mm and 0.6 mm) and two contact times (6 and 12 h)
In this study, handmade funnels made from plastic bottles (355 mL) were used, then 4 g of activated carbon from jícaro husk (2-4 mm for each funnel) were weighed; 200 mL of each of the previously prepared As solutions (10, 25 and 50 µg As/L, was added to each funnel (quickly stirred with a glass rod). After 6 hours of contact, filtration was carried out. This same procedure was repeated for 12 h of contact (they were performed in duplicate) The final concentrations of the liquid remnant were determined using the hydride generation technique.
This same procedure was applied for tests with activated carbon with a diameter (0.6 mm) at 6 and 12 h contact.
2.1.2. Preparation of half-inch (1/2 ”) rusty iron nails
The nails of 1/2” inch non-galvanized irons were placed in a corduroy with water, leaving them in contact for 2 weeks. After two weeks the nails were removed from the water and left in the sun for two days, with the objective of eliminating any water particle (AMEC, 2015) This procedure was carried out to produce iron oxides, which was used to retain arsenic.
2.1.3. Determination of arsenic removal by continuous removal with iron oxides and activated carbon (0.6 mm and 6 h)
In this part, 4 g of previously oxidized ½ inch nails were weighed, which were deposited in each funnel. Subsequently, 200 mL of each of the solutions with concentrations of 14, 29 and 54 µg As/L were added. After 6 hours of contact, the liquid was separated, then transferred to other funnels, previously prepared with 4 g of activated carbon (0.6 mm). The solutions were left in contact with the activated carbon for 6 h. After the contact time, the liquid phase was separated for further analysis (these determinations were made in triplicate).
This methodology was taken from the Kanchan filter developed by researchers from the Massachusetts Institute of Technology (MIT), the Nepal Environment and Public Health Organization (ENPHO) and the Rural Water and Sanitation Supply Support Program (RWSSSP) of Nepal, based on slow filtration filters and iron hydroxide (Ngai, Dangol, Murcott, & Shrestha, 2005).
Once the final concentrations of arsenic were obtained after being subjected during a period of contact with the adsorbent media, the percentages of arsenic removal were calculated by means of activated carbon produced artesanally based on jícaro shell with sizes 2 - 4 mm; 0.6 mm and at different contact times (6 and 12 h):
Equation No. 1. To calculate the removal percentages
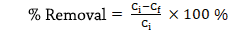
Where:
Ci: Initial concentration of As (µg As/L)
Cf: Final concentration of As (µg As/L)
2.2. Description of the analytical methodology
In the laboratory of Metallic Contaminants of CIRA / UNAN Managua, the concentrations of total arsenic to the samples obtained in each test were determined. The determination was carried out by atomic absorption spectrometry (Varian Spectr AA-240 FS spectrometer) with the hydride generation technique (VGA 77). The detection limit of the method is 0.99 µg As/L for the analyzed matrix (SMWW, 2005).
3. Results and discussion
3.1. Arsenic removal after 6 and 12 h contact time with activated carbon (2-4 mm)
3.1.1. Test with contact time of 6 h
Graph 1 shows the averages obtained from the final arsenic concentrations of 10 µg As/L, 24 and 50 µg As/L (initial of 10, 25 and 50 µg As/L), for a percentage of arsenic removal of 3%, 5 and 0.5% respectively.
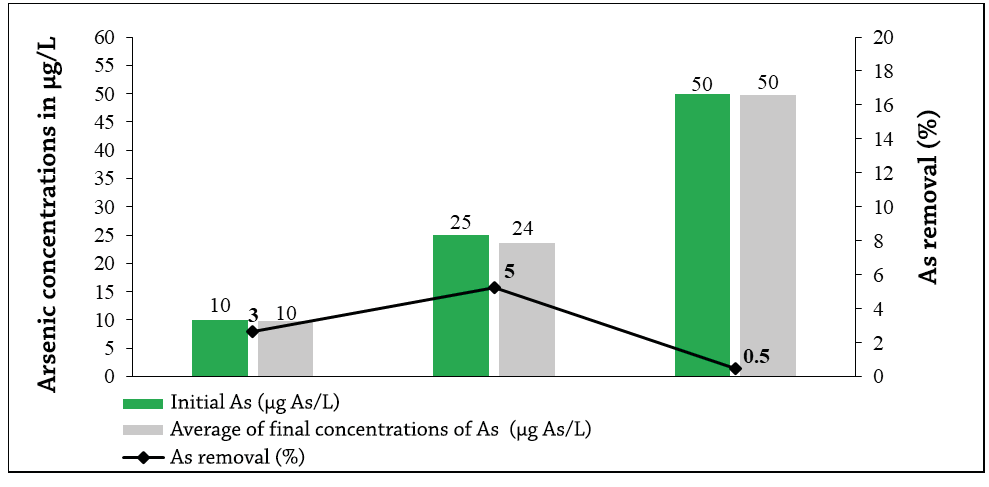
Graph 1. Final concentrations of As (µg / L) after being exposed for 6 hours of contact with activated carbon of 2-4 mm size.
The highest removal is for the concentration of 25 µg As/L and the lowest for that of 50 µg As/L, possibly because the pores of the activated carbon were saturated by decreasing the contact area between the analyte and the adsorbent agent. The ability of an activated carbon to retain a given substance is not only given by its surface area, but by the proportion of pores whose size is adequate (Ures Rodríguez, Suárez López, & Jácome Burgos, 2015)
3.1.2. Test with contact time of 12 h
The final concentration values of Arsenic removal after the 12 h contact time, analyzed under the same concentrations previously established for the 6 h tests, reflected similar removal results 9, 23 and 50 µg As/L as shown in Graph 2. The resulting values in percentages correspond to 6, 6 and 1% respectively, being the highest removals obtained for the concentrations of 10 and 25 µg As/L.
Based on the results obtained from the two contact times of 6 and 12 h, the mean difference test was applied, which allowed us to verify that there is no significant difference between the adsorbed concentrations in the two contact times (P > 0.05) .
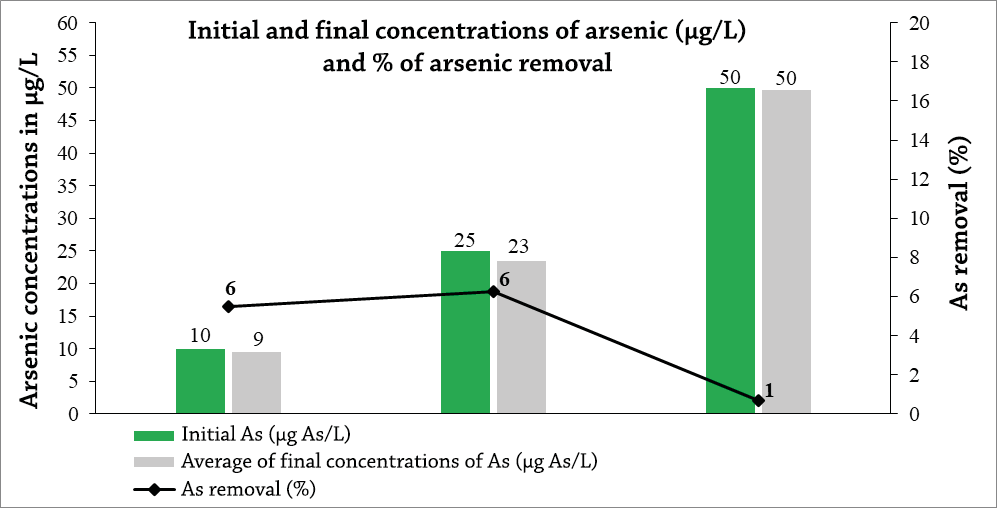
Graph 2. Final concentrations of As after being exposed for 12 hours of contact with activated carbon of 2-4 mm size.
3.2. Arsenic removal after 6 and 12 h contact time with activated carbon (0.6mm) produced by ADECAB
3.2.1. Test with contact time of 6 h
In general, the results obtained for arsenic removal using three different concentrations (10, 25 and 50 µg As/L), in 6 hours of contact with 0.6 mm activated carbon, were higher compared to those obtained with a size of 2-4 mm, obtaining final concentrations of 9, 23 and 43 µg As/L, respectively (Graph 3). The highest removal percentage was obtained at 50 µg As/L with 14%.
The low adsorption of activated carbon may be due to the size of its pores, macropores and mesopores may largely prevail, since this is a very important and decisive property of adsorbent materials is the size of its pore, most of the total surface area found in micropores, therefore, these are the most available adsorption centers for molecules with relatively low molecular weight (Ures Rodríguez, Suárez López, & Jácome Burgos, 2015).
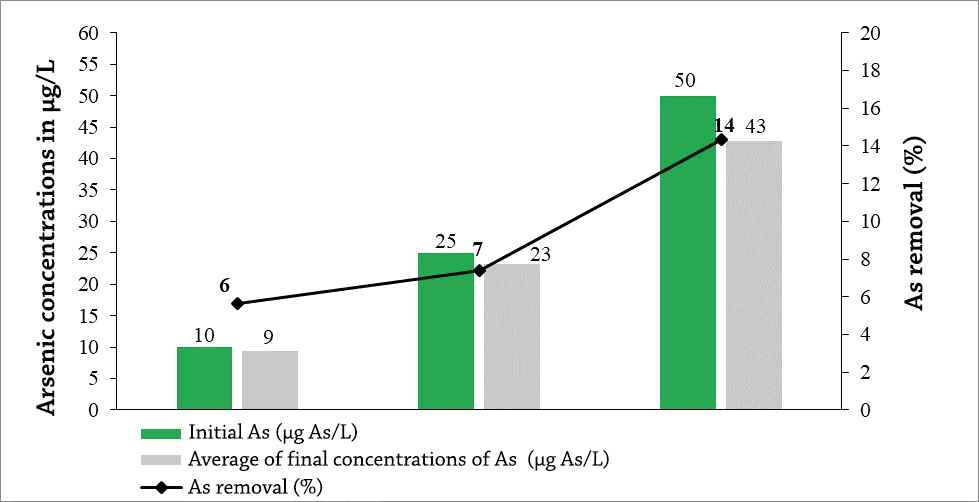
Graph 3. Final concentrations of As after being exposed for 6 hours of contact in activated carbon with a size of 0.6
3.2.2. Test with contact time 12 h
The final concentrations of the three arsenic solutions tested (10, 25 and 50 µg As/L) when subjected to contact with activated carbon of 0.6 mm for 12 h, were 9, 23 and 43 µg As/L (Graph 4). The removal percentages correspond to 7, 10 and 15%, respectively. Reaching the highest removal percentage in the 50 µg As/L solution.
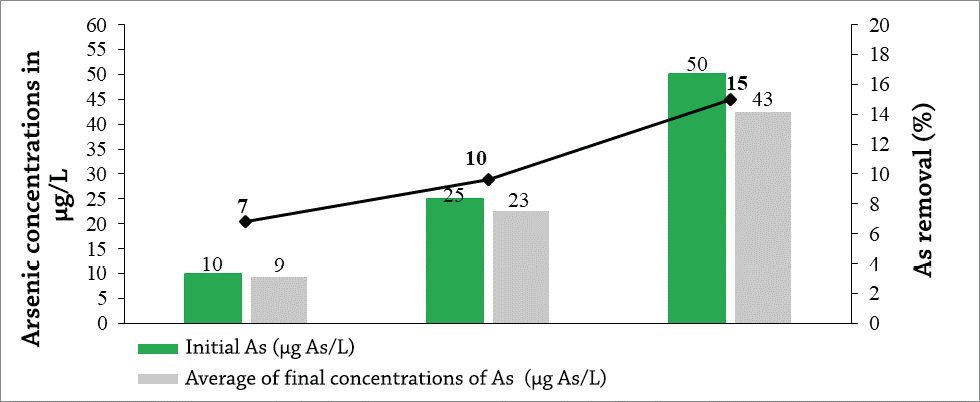
Graph 4. Final concentrations of As after being exposed for 12 hours of contact in activated carbon with a size of 0.6 mm.
Compared to the sizes of activated carbon, the adsorption capacity is greater than 0.6 mm, this is perhaps due to the fact that adsorption is generally considered proportional to the specific surface area of contact, so the more finely divided and more porous the carbon is activated, the higher the expected adsorption performance per unit of adsorbent weight as indicated (Ures Rodríguez, Suárez López, & Jácome Burgos, 2015).
However, the mean differences test showed that there is no significant difference between the adsorbed concentrations in the two contact times P > 0.05, so that the contact times do not significantly influence the removal percentages.
3.3. Arsenic removal after 6 hours of contact with iron oxides and activated carbon (0.6 mm)
The final arsenic concentrations of the three solutions tested with rusty iron nails (½ inch nails) and artisanal activated carbon of 0.6 mm (14, 29 and 54 µg As/L) produced final concentrations of 6, 14 and 22 µg As/L, respectively (Graph 5).
In the sequential treatment with iron oxides and activated carbon, the highest arsenic removal in the highest concentration solution (54 µg As/L) was given with 58%. This high removal rate could be due to a surface complexing reaction between iron oxides and arsenic thus forming a floc, followed by the adsorption of these flocs (Amirtharajah & O’Melia, 1990), where these iron particles are loaded with arsenic were retained by activated carbon (Ngai, Dangol, Murcott, & Shrestha, 2005).
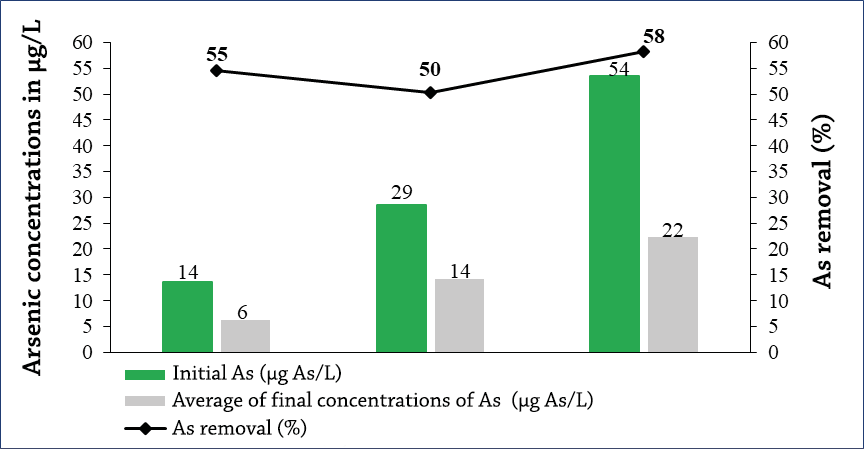
Graph 5. Final concentrations of As after undergoing slow filtration by iron oxides and then exposed for 6 h of activated carbon contact with size 0.6 mm.
The larger molecules are adsorbed on activated carbon better than smaller molecules, this is because the adsorption occurs in the micros (size less than 2 nm) and mesopores (2-50 nm) of the activated carbon; mesopores are of greater predominance and that is why the flocs adsorb more easily (Gregg & Sing, 1982).
4. Conclusions
The highest percentages of arsenic removal were obtained for sequential treatment with iron oxides and artisanal activated carbon of 0.6 mm and with a contact time of 6 hours (54, 50 and 58%, respectively, for the three concentrations treated). No significant differences were detected between the results obtained during the contact times and sizes of activated carbon.
Acknowledgements
To the Funds for Research Projects (FPI), granted by the Universidad Nacional Autónoma de Nicaragua (UNAN-Managua) and to the Center for Research in Aquatic Resources of Nicaragua (CRAR/UNAN-Managua) for co-financing this research.
Bibliography
Altamirano E, M. (2005). Distribución de la contaminación natural por arsénico en las aguas subterráneas de la subcuenca suroeste del Valle de Sebaco, Matagalpa-Nicaragua. Managua: CIRA/UNAN-Managua.
AMEC. (04 de Mayo de 2015). Componentes del filtro para la remoción de Arsénico. AMEC, Managua, Nicaragua.
Amirtharajah, A., & O’Melia, C. (1990). Coagulation processes: Destabilization, mixing and flocculation (4th ed.). (F. W. Pontius, Ed.) Nueva York, N.Y., EE.UU: McGraw-Hill.
DeMarco, M. J., SenGruta, A. K., John, E., & Greenleaf, J. E. (2003). Arsenic removal using a polymeric/inorganic hibrid sorbent. Water Research 37,. 164-176.
Esparza, M. C. (2006). Presencia de arsénico en el agua de bebida en América Latina y su efecto en la salud pública. International Congress: Natural Arsenic in Groundwaters of Latin America. Mexico.
Ferreccio, C., González, C., Milosavjlevic, V., Marshall, G., Sacha, A. M., & Smith, A. H. (2000). Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 11(6). 676-679.
Gregg, S. J., & Sing , S. W. (1982). Adsorption, Surface Area and Porosity. London Academic Press.
Han, B., Runnells, T., & Wickramasinghe, R. (2002). Arsenic renoval from drinking water by flocculation and microfiltration, Desalination 145. 293-298.
Hughes, M. (2002). Arsenic toxicity and potencial mechanisms of action. Toxicology letters. 133,, 1 - 16.
Kapaj, S., Peterson, H., Liber, K., & Bhattacharya, P. (2006). Human Healh Effects from arsenic poisoning-a review. Journal of Environmental Science and Health. Part A 41,. 2399-2428.
Mohan, D., & Pittman, ,. C. (2007). Arsenic removal from water/wastewater using adsorbents-a critical review. Journal of Hazardous Materials 142. 1-53.
Montero Campos, V., Quesada Kimsey, J., Ledezma Espinoza, A., & Sandoval Mora, J. A. (2010). Determinación de arsénico en abastecimientos de agua para consumo humano de la provincia de Cartago, Costa Rica.
Ngai, T. K., Dangol, B., Murcott, S. E., & Shrestha, R. R. (2005). Kanchan Arsenic Filter.Massachusetts Institute of Technology (MIT) and Environment and Public Health Organization (ENPHO). Kathmandu, Nepal. Kathmandu, Nepal.
OMS. (1995). Organizácion Mundial de la Salud.
SMWW. (2005). Standard Methods for the Examination of Water and Wastewater. American Public Healh Association (APHA), 21 .
UNICEF. (2002). MONITOREO Y ATENCIÓN DE INTOXICADOS CON ARSÉNICOEN EL ZAPOTE, MUNICIPIO DE SAN ISIDRO, DEPARTAMENTO DE MATAGALPA, NICARAGUA 1994-2002. Managua: DRA. ALINA GÓMEZ C. Obtained from https://studylib.es/doc/6457827/monitoreo-y-atenci%C3%B3n-de-intoxicados-con-ars%C3%A9nico-en-el-za...
UNICEF. (2004). Contribución al Estudio de Cinco Zonas Contaminadas Naturalmente por Arsénico en Nicaragua. Managua: UNICEF - Nicaragua.
Universidad Politecnica de Sevilla. (2002). MANUAL DEL CARBON ACTIVO. Retrevied in 2016 from http://www.elaguapotable.com/Manual%20del%20carb%C3%B3n%20activo.pdf
Ures Rodríguez, P., Suárez López, J., & Jácome Burgos, A. (May 2015). ADSORCIÓN EN CARBÓN ACTIVO (FT-TER-002). INDITEX.
USEPA. (2007). Treatment Technologies for Arsenic Removal.