ISSN 2410-5708 / e-ISSN 2313-7215
Year 13 | No. 37 | June - September 2024
© Copyright (2024). National Autonomous University of Nicaragua, Managua.
This document is under a Creative Commons
Attribution-NonCommercial-NoDerivs 4.0 International licence.
Molecular testing for breast cancer: Systematic review of availability in Latin American and Caribbean countries
https://doi.org/10.5377/rtu.v13i37.18176
Submitted on november 16th, 2023 / Accepted on May 23th, 2024
Jackeline de Fátima Martínez González
National Autonomous University of Nicaragua, Managua.
Polytechnic Institute of Health, POLISAL
Marianela Corriols Molina
National Autonomous University of Nicaragua, Managua.
Faculty of Medical Sciences
Section: Health and Social Services
Scientific research article
Keywords: Oncotype, MammaPrint, Breast Cancer Index, EndoPredict, Prosigna, Genetic testing, Genomic testing.
ABSTRACT
To systematize the availability in clinical practice of genomic and genetic tests for breast cancer (BrCa) in Latin American and Caribbean (LAC) countries.
Methods: The PRISMA 2020 framework was used for the literature review and web search, published in Spanish and English, using an explicit method to compile and synthesize the findings, between January 2010 and December 2023.
Results: Five genomic tests used in clinical practice were found: OncotypeDx, MammaPrint, Breast Cancer Index (BCI), EndoPredict, and Prosigna. These tests are offered in 14 of 48 (27%) LAC countries. The most provided test is Oncotype present in 11/14 countries (79%), followed by EndoPredict in 9/14 countries (64%), and the least offered is BCI present in 1/14 countries (7%). Of the available genetic tests, ten were found to analyze BRCA 1/2 genes and fourteen for genomic panels, offered in 18 of 48 countries (38%) in LAC.
Discussion: Molecular tests for breast cancer have changed the management of this neoplasm in the countries that have incorporated them into clinical practice. Genomic tests have made it possible to identify patients who do not require chemotherapy. Genetic testing is available in those countries that have investigated for many years the frequency of susceptibility genes in women at risk. LAC countries should carry out cost-benefit studies on the use of molecular testing and promote evidence-based guidelines on the prevention of HSCLC and the therapeutic management of patients with HSCLC.
INTRODUCTION
Molecular tests are now available for the analysis of genetic and genomic variants that are part of the clinical management of patients with breast cancer (BrCa). Genetic testing identifies inherited mutations in specific genes, while genomic testing generally analyzes the sequence or expression of groups of genes, large fragments of the genome, and even the entire genome. (Linchar et al., 2015) In clinical practice, genetic variant analysis is targeted to patients with family and personal history of BrCa or other early onset tumors to determine the existence of hereditary cancer syndrome, on the other hand, genomic expression signatures are offered to patients with early-stage estrogen receptor-positive (ER+) BrCa, as prognostic for endocrine therapy and predictive of chemotherapy benefit. (Linyton et al., 2019) (Griguolo et al., 2022)
Azim et al., (2012) evaluated the medical utility of six genomic tests developed for early BrCa: OncotypeDx, MammaPrint®, Genomic Grade Index (GGI), PAM50, Breast Cancer Index (BCI), and EndoPredict. They performed a critical review of available studies on these tests to determine analytical validity, clinical validity, and utility. They found that OncotypeDx and MammaPrint® have analytical validity and clinical validity, but none of the tests evaluated demonstrated clinical utility.
Linchar et al., (2015), specify that the sample type for DNA analysis requires a blood sample or a tissue sample (such as saliva or cheek swab). They indicated that one of the guidelines for BRCA testing includes high-risk individuals with a personal or family history of BrCa or ovarian developed at a young age (less than 50 years), established by the American College of Medical Genetics and the United States Preventive Services Task Force (USPSTF). They concluded that genetic testing will become increasingly important in the prevention, diagnosis, and treatment of hereditary MHCA and that the incorporation of multigene panels into clinical practice will allow an increasing number of genes to be routinely tested for mutations associated with this cancer.
Vargas-Aguilar et al, (2018) identified six clinically available genomic expression signatures as prognostic markers: IHC4 assay, OncotypeDX, EndoPredict, Prosigna-PAM50, MapQuant Dx, The Breast Cancer Index, indicating that the first generation of prognostic signatures (Oncotype DX, MammaPrint, Genomic Grade Index) predict recurrence at five years and subsequent tests (Prosigna, EndoPredict, Breast Cancer Index) possess better prognostic value for recurrence and are predictive of early relapse. They report that there are no useful prognostic genetic tests for hormone-negative tumors, nor predictors of response to treatment.
Litton et al., (2019), studied molecular testing in BrCa, evidencing its significant evolution, as initial tests sequenced only BRCA 1 and 2 genes. With the development of next-generation sequencing, multiple genes can now be analyzed simultaneously, saving time for patients waiting for results before making treatment decisions. About gene expression assays, they explain that before the advent of genomic signatures, there were no adequate tools to select patients for treatment with adjuvant chemotherapy, with a large evidence base (meta-analyses, retrospective and prospective studies, retrospective and prospective comparative observational studies, and prospective and observational studies), prospective comparative observational studies and retrospective observational studies) showing the clinical utility of the Oncotype DX, MammaPrint, EndoPredict, Prosigna PAM50, and Breast Cancer Index tests can be used in cases of node-negative ER+ breast cancer to guide decisions about adjuvant therapy.
Lulu et al, (2021) studied the Oncotype DX, MammaPrint, Prosigna, and Breast Cancer Index molecular assays to report their prognostic (recurrence/survival) or predictive (response to treatment) value. They found that all four assays provide prognostic value for the risk of recurrence and there is sufficient evidence for the therapeutic value of OncotypeDX (Predictive of chemotherapy benefit) and Breast Cancer Index (Predictive of prolonged endocrine therapy).
Bernet et al. (2022) conducted a review of the 4 most widely used genomic expression signatures in clinical practice in Spain: MammaPrint, OncotypeDX EndoPredict and Prosigna PAM50. They indicated that, due to the nature of breast cancer, the use of these tests has become an increasingly used tool for the correct stratification, prognosis, and treatment of cancer. They expressed that before the advent of genomic tests, there was no adequate tool to identify patients who could benefit from chemotherapy and those who could not, since, historically, adjuvant chemotherapy was recommended for patients with tumors larger than 1 cm and with lymph node involvement, considered in many cases as “overtreatment”. The authors describe in this study the differences between them both in their development and in their technical and clinical validation.
The objective of this research is to systematize the availability in clinical practice of genomic tests that allow defining the risk of recurrence and treatment prediction in patients with early-stage BrCa, as well as the availability of genetic tests that determine pathogenic variants (PV) of susceptibility to BrCaH, in Latin American and Caribbean (LAC) countries.
METHOD
The PRISMA 2020 framework (Page et al., 2021) was used as a reference to conduct a systematic review of the literature and web pages on the subject, using an explicit method to compile and synthesize the findings that address the following questions: Which LAC countries have genomic tests for BrCaH and genetic tests for BrCaH? and What are these genomic and genetic tests? The events studied are the existence of genomic tests and genetic tests in LAC for BrCa and the clinical applicability of these tests, and the outcome is the proportion of countries that offer molecular tests for BrCa and the availability of the service of these tests in each country.
As a source of information, a bibliographic search was carried out in databases in PubMed, Scielo, and websites of digital platforms of laboratories that offer genetic tests for BrCa in LAC, published in Spanish and English between January 2010 and December 2023. The following terms were used: “Pruebas genéticas”, “Pruebas genomicas”, “Oncotype”, “Mammaprint”, “Endopredict”, “Prosigna”, “Breast Cancer Index”, with a specific search using the name of each of the 48 countries or territories included in the study. Titles and abstracts of articles were reviewed, the inclusion criteria being: randomized studies, meta-analysis, cohort studies, case-control studies, systematizations, and cross-sectional studies. Series and case reports and studies outside the period and in languages other than English or Spanish were excluded.
To evaluate the availability of the tests, in addition to the bibliographic review from secondary sources, a direct search was made through telephone calls, text messages, and e-mails to laboratories and institutions to confirm the existence or not of the tests. In the case of information from laboratories and institutions, all those accessible on the web were included.
Regarding the data extraction process: one reviewer (JG) collected the information related to the topic, which was validated with the second reviewer (MC). The information collected was organized in tables according to the specific objectives. The existing tests were grouped by country, classifying them into first and second-generation tests.
To synthesize the information, a flow chart, a table of genomic expression tests for BrCa, and a table of availability of genetic tests for BrCaH in LAC were elaborated.
In the search process, both investigators reviewed and selected independently at the beginning and then jointly. In case of discrepancy, inclusion/exclusion criteria were reviewed jointly.
In terms of limitations, it is recognized that there may be laboratories and institutions in the countries studied that offer genetic testing and gene expression testing for BrCa that do not have web-based digital platforms.
RESULTS
We reviewed 356 scientific articles, identifying 186 on genomic and genetic testing for BrCa, of which 25 were included because of their relevance to the topic, seven of them identified in previous reviews, and 19 were found in this review. A total of 2,821 websites were reviewed, selecting 56 web publications to be included in the systematization.
Genomic tests
Five genomic tests are used for expression analysis of multiple genes that determine the probability of distant metastasis and prognosis of adjuvant treatment in BrCa (recurrence and prediction): OncotypeDx (Syed Y. 2020), (Lulu et al., 2021), (American Cancer Society, 2023), (Genomic Health, Inc.), MammaPrint® (Sánchez-Forgach, et al., 2017), (Brandão et al., 2019); EndoPredict (Longwood Diagnóstica), (Dubsky et al., 2013), (Muller et al., 2013), (Sestak et al., 2019); Breat Cancer Index (BCI) (Zhang, Y. et al., 2013), (Sgroi et al., 2013), (Sanft et al., 2015), (NOEGenomic, 2023) and Prosigna (Kelly et al., 2012), (Dowsett et al., 2013), (Gonzalez et at., 2015). These are described below:
OncotypeDX: Analyzes quantitative expression levels of 21 genes through RT-PCR, of which 16 are cancer-related genes and 5 are reference genes. It is analyzed from RNA extracted from a paraffin-embedded tumor sample. (Lulu et al 2021) It is indicated for patients with early-stage BrCa, estrogen receptor-positive ER (+), human epidermal growth factor HER2 (-) with LN (-), or up to three LN (+). (American Cancer Society, 2023).
This assay calculates a recurrence score based on the weighted expression of each gene at high risk, intermediate risk, and low risk, extensively validated by clinical studies worldwide. (Bernet et al., 2022) The higher this score, the higher the risk of distant recurrence and the greater the likelihood that the patient will benefit from chemotherapy, while a low risk indicates the opposite. It is currently the only validated multigene assay for predicting chemotherapy benefits as well as prognosis (Syed Y. 2020, Genomic Health, Inc).
MammaPrint: Analyzes 70 BrCa-associated genes using DNA microarrays, designed to assess 10-year recurrence in patients with early BrCa, independent of conventional clinical and pathological factors. A low-risk score allows waiving the use of adjuvant chemotherapy (AGENDIA, 2023, Sanchez-Forgach, et al., 2017). Its use as a prognostic biomarker has been widely validated, both retrospectively and prospectively. However, its value as a predictive and clinically useful tool remains controversial (Brandão et al., 2019).
EndoPredict: This molecular test is based on the quantification of mRNA levels of 12 genes (eight cancer genes, three reference genes, and one control gene) by quantitative RT-PCR in formalin and paraffin-embedded (FFPE) tissue. The score allows for establishing a low-risk group or a high-risk group for recurrence (Longwood Diagnostics, Dubsky et al., 2013, Sestak et al., 2019). It prognoses and predicts the risk of metastasis of patients with ER (+), HER2 (-) BrCa treated only with endocrine therapy, and unlike previous tests this one includes clinicopathological factors such as tumor size and nodal status (Muller et al., 2013, Longwood Diagnosis, Myriad Genetics).
Breast Cancer Index (BCI): analyzes gene expression of eleven genes via RT-PCR, incorporates a biomarker associated with tumor response to endocrine therapy and a biomarker associated with the cell cycle, providing a quantitative and objective molecular assessment of tumor proliferative status. (Zhang, Y. et al., 2013, Sanft et al., 2015) It provides information on a patient’s individualized risk of distant recurrence and prediction of the likelihood of benefit from prolonged (greater than 5 years) endocrine therapy. The test is designed for women diagnosed with early-stage invasive hormone receptor-positive (HR+), LN (-), or 1-3 LN (+) nodes who do not have distant recurrence (Sgroi et al., 2013, NEOGenomic, 2023).
Prosigna (PAM50): The assay analyzes gene expression of 50 classifier genes and five control genes to identify intrinsic subtypes (luminal A/B, HER2-enriched, basal-like) via RT-PCR of tumor tissue in FFPE. This assay estimates the risk of distant recurrence in postmenopausal women with early-stage HR (+) hormone receptors 5-10 years after diagnosis and after 5 years of treatment with hormone therapy. (Kelly et al., 2012, Dowsett et al., 2013) The results of the analysis are reported in three risk categories with a score from 0-100: Cancer cases with LN (-) are classified; as low (0-40), intermediate (41-60), and high (61-100) risk. Cancer cases with LN (+) are classified as low (0-40) and high (41-100) risk. (Gonzalez et al., 2015) (Table 1).
Availability of genomic tests in LAC.
Laboratories offering these tests were found in 14 of 48 countries (27%) in LAC. The most offered test is Oncotype first generation, present in 11 out of 14 countries (79%), followed by EndoPredict second generation, present in 9 out of 14 countries (64%). MammaPrint and Prosigna are present in 8 out of 14 countries (57%) respectively. The least offered is BCI, which is available in only 1 of 14 countries (7%).
The countries with the highest web visibility on the availability of these tests are Mexico: ABC Medical Center, Genetics Services, GeneLab, Patiacan, s.f, Senocuidado, Milenia Labs and Syn Lab s.f; Argentina: OMICS Exact Sciences, One Light Solution, s.f, Varifarma, Argenetics, s. f; Brazil: Fleury Genomica, Agendia, NEOGenomic; Syn Lab n.d.; Chile: South Genetics, One Light Solution, n.d., Varifarma; Colombia: Amarey (n.d.), GenCell, Genética Avanzada, Syn Lab n.d.; Peru: Centro Oncológico Aliada, GeneCode S.A.C., Varifarma and Syn Lab; Uruguay: South Genetics, One Light Solution, n.d., Longwood and Varifarma.
Two tests were identified as available in Paraguay: South Genetics-Oncotype, Varifarma; Dominican Republic: South Genetics, Medipath Instituto de patología molecular; Puerto Rico: Puerto Rico Pathology; Ecuador: Varifarma, Syn Lab n.d. Laboratories offering one of the tests were found in Bolivia: Instituto BioClínico Cruceño Ltda Análisis Clínicos & Diagnostico; Guatemala: Integra Cáncer Instituto; Honduras: Fertilab. (Table 2).
No evidence of the availability of these tests was found in Central America (El Salvador, Nicaragua, Costa Rica and Panama); The Caribbean (Cuba, Bahamas, Barbados, Haiti, Cayman Islands, Trinidad and Tobago, Jamaica, Dominica, Guyana, Suriname, Antigua and Barbuda, Grenada, Belize, St. Kitts and Nevis, St. Vincent and Grenadines, St. Lucia, Aruba, Guadeloupe, Turks and Caicos Islands, Virgin Islands, Martinique, St. Barthelemy, Anguilla, Netherlands Antilles, Bonaire, Curaçao, Bermuda, Falkland Islands, and Monserrat).
Some interesting facts: In Mexico MammaPrint is included in the Clinical Guides for treatment against BrCa; in Argentina, Oncotype has insurance coverage; in Colombia OncotypeDx and MammaPrint are included in the clinical practice guide; in Venezuela OncotypeDx, MammaPrint, and Prosigna are included in the Venezuelan Guide for the diagnosis and treatment of BrCa, however, they indicate that they are of limited use due to their high cost and availability, but they do have sample collection for sending abroad for the required analysis. (Ambios, n.d.)
Genetic tests
The genetic tests currently available for BrCaH are directed to the analysis of BRCA 1/2 genes and non-BRCA genes (gene panels).
For the analysis of BRCA 1/2 genes, ten tests were found: myBRCA; BRCA1 and BRCA2 Plus; BRCA1 and BRCA2 Combi; BRCA1 and 2 (8838); BRCA1 and BRCA2; BRCATIX; BRCA 1/ 2; BRCA analysis; BRCA 1 and 2 NGS Express; and BRCA Panel. These tests are offered by South Genetics, CentoGen, Genómica Medica- Laboratorio de Diagnóstico Molecular, ROSSI, Milenia Labs, Patiacan, s.f, and Genetix, Quest Diagnostics (Table 1).
Fourteen gene panel tests were found that include BRCA gene analysis: Oncorisk; myBRCA HiRisk; CentoBreast; CentoCancer; CentoCancer Comprehensive; BRCA Plus (C8836); Cancernext (8824); BRACATIX Plus; Oncotix; Onocopanel; SENTIS Breast and Ovarian; BRACA +16; BreastDetect; and BRCA Plus. These tests are performed by Genetic Services, South Genetics, CentoGen, Genómica Medica-Laboratorio de Diagnóstico Molecular), Genetix, ADN Uruguay, SynLab, Biocells Genomics, and Quest Diagnostics.s, f. (Table 3).
Availability of genetic testing in LAC
In 18 of 48 countries (38%) in LAC, these tests are available for women at risk of BrCaH, ranging from genetic tests for the analysis of familial VP (BRCA 1/2) to the analysis of genetic panels that include up to 118 genes related to the risk of common hereditary cancers, such as BrCa, ovarian, colorectal, pancreatic and others. (Table 2).
Of these tests, the most commercialized in the region are myBRCA (analysis of BRCA 1/2 genes) and myBRCA HiRisk (analysis of 26 genes, including BRCA1/2), marketed in Mexico, Argentina, Colombia, Ecuador, Paraguay, Peru, Uruguay, Bolivia, Venezuela, Panama, and the Dominican Republic. The countries with the highest visibility in terms of supply through digital platforms of different genetic tests are Mexico, Argentina, and Colombia. In some countries, there is information to the public about the cost of these tests (between $1500 and $2000).
In Central America, the following countries offer counseling and tests for BRCA 1 / 2 gene analysis and genetic panels: Costa Rica (Hospitales Clínica Bíblica and Metropolitano), Guatemala (Integra Cancer Institute), Honduras (Laboratorios Centro Ginecológico), El Salvador (Fertilab s.f.) and Panama (South Genetic and Vida Tec). In Nicaragua, these analyses are not offered; only a sample is taken to be sent abroad, at an approximate cost of US$3,000 (Vivian Pellas Hospital).
No evidence of the availability of these tests was found on websites in Caribbean countries and territories in Belize, Cuba, Bahamas, Barbados, Haiti, Cayman Islands, Trinidad and Tobago, Jamaica, Dominica, Guyana, Suriname, Antigua and Barbuda, Grenada, Belize, St. Kitts and Nevis, St. Vincent and Grenadines, St. Lucia, Aruba, Guadeloupe, Turks and Caicos Islands, Virgin Islands, Martinique, St. Barthelemy, Anguilla, Netherlands Antilles, Bonaire, Curaçao, Bermuda, Falkland Islands, and Monserrat.
Limitations of genetic testing for BrCaH
Four studies discuss the limitations of the use of genetic testing for BrCaH. These include: a) indications and interpretations of VP of less common non-BRCA genes are not established; b) testing costs are not standardized and depend on the laboratory and health insurance coverage. (Valencia et al., 2017); c) lack of financial resources impacts the performance of BRCA 1/2 testing in the at-risk population. (White et al., 2018); d) geographic access to services, potential provider discrimination and bias, and lack of education and awareness of both patient and provider are barriers to the identification of VP carriers. (Reid et al., 2022) and e) shortage of genetic counselors, and lack of physician knowledge on how to evaluate, identify, and refer patients to genetic counseling are factors that limit optimization of genetic testing. (DeTroye, A. et al., 2022)
DISCUSSION
This is the first study to provide information on the availability at the LAC level of molecular tests (genetic and genomic), both for diagnosis and for prognosis of recurrence and prediction of treatment for BrCa. Advances in molecular biology have changed the management of this cancer in countries that have incorporated these tests into clinical practice. In countries that do not have these tests, treatment for women diagnosed with breast cancer is determined by clinicopathological factors and patients receive a combination of endocrine therapy and chemotherapy, which in many cases is not required, in addition to the uncertainty of the duration of endocrine treatment. According to Litton et al., (2019) and Bernet et al., (2022), this dilemma was resolved with the development of genomic expression testing, as, these tests identify patients who would not benefit from chemotherapy.
However, clinicopathologic factors remain highly relevant in the management of MCA, therefore, genomic tests that incorporate these risk factors become more valuable and should be further considered for integration into clinical practice. Of the five genomic tests studied OncotypeDx, EndoPredict, and Prosigna are the ones that incorporate these factors.
Concerning genetic tests for breast cancer, the availability of a variety of tests in the Latin American region is evident. Mexico, Argentina, Colombia, and Chile are the countries with the greatest offer, related to what was described by Martínez and Corriols (2023) who demonstrated that these countries concentrate the greatest proportion of biomedical research on genes with VP related to BrCaH in the last two decades. Conversely, countries that do not research the subject do not have this evidence.
This study is the first systematic review of the availability and use of genetic and genomic testing for BrCa in LAC, demonstrating the importance and need to continue studying it, mainly in those countries where the prevalence, the genes involved, and the inherited PV are unknown. The challenge of increasing access to these tests in less developed countries remains, related by some authors to limitations of financial resources, lack of insurance coverage, lack of personnel training, lack of support structures, education, and genetic counseling for patients and families, as well as development and implementation of health policies. I conclude that the approach to HmAmCa among LAC countries is unequal and that knowledge on this subject is still insufficient; the countries that have done the most research are those that currently have the molecular tools that allow better management of HmAmCa.
It is recommended that LAC countries carry out cost-benefit studies on the use of molecular tests in the prevention of breast cancer and the therapeutic management of patients with breast cancer and promote evidence-based guidelines with interventions for screening, diagnosis, and treatment of breast cancer, including molecular tests in health sector care plans.
Regarding the limitations of the study, the possible underreporting of information on the availability of molecular tests for breast cancer due to a lack of response from some laboratories consulted at the international level and the possible omission of providers who do not use websites when searching for information.
Financing
This study was financed by the UNAN-Managua.
Conflict of interest
The authors declare that they have no conflict of interest.
ANNEXES
Figure 1
Flowchart for information selection
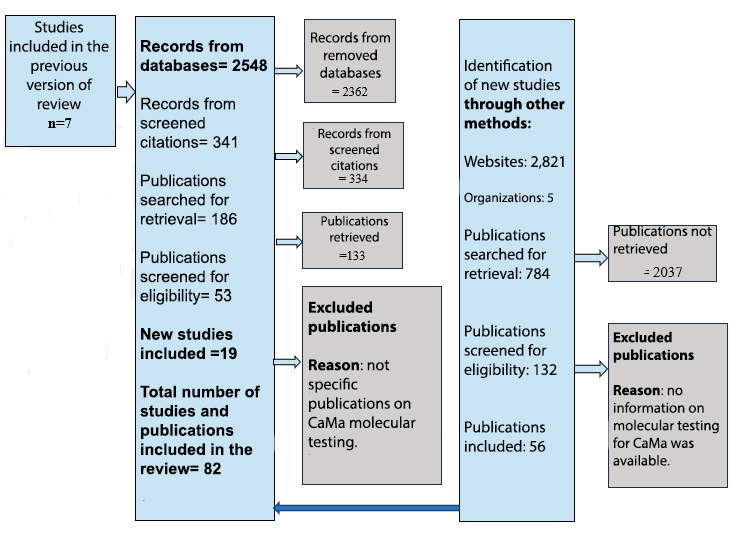
Source: PRISMA 2020 Template
Table 1
Specifications of the genomic tests for BrCa available in LAC
|
Variable |
Oncotype DX |
MammaPrint |
EndoPredict |
Breast Cancer Index |
Prosigna |
|
Análisis |
21 genes |
70 genes |
12 genes |
11 genes |
50 genes |
|
Technique |
RT-PCR Quantitative |
DNA-microarrays |
RT-PCR Quantitative |
RT-PCR Quantitative |
RT-PCR |
|
Indications |
Early stage, RE (+), HER2 (-), LN (-), or up to three LN (+). |
Early stage, independent of conventional clinical and pathologic factors |
Early stage, RE (+), HER2 (-), treated with endocrine therapy alone, tumor size and nodal status |
Early stage CaMa invasive, HR (+), LN (-) o 1-3 LN (+) |
Early stage Postmenopausal, RH (+), LN (-), LN (+) |
|
Clinical utility |
Prediction and prognosis |
Prediction |
Prediction and prognosis |
Prediction and prognosis |
Prediction |
|
Type of sample |
Formalin-fixed tumor tissue |
Source: Own elaboration
Table 2
Availability of genomic expression tests for BrCa in LAC.
|
First generation |
Second generation |
||||
|
Country |
Oncotype |
MammaPrint |
EndoPredict |
BIC |
Prosigna PAM50 |
|
México |
Centro médico ABC |
Genetics services |
GeneLab (Patiacan, s.f.) |
- |
Senocuidado Milenia Labs Syn Lab s. f |
|
SOUTH AMÉRICA |
|||||
|
Argentina |
OMICS Exact Scencies |
One Light Solution, s.f. |
Varifarma |
Argenetics, s.f. |
|
|
Brazil |
Fleury Genomica |
Agenda |
NEOGenomic |
Syn Lab s. f |
|
|
Chile |
South Genetics |
One Light Solution, s.f. |
Varifarma |
- |
South Genetics |
|
Colombia |
Amarey. (s.f.) |
Gencell Genética avanzada |
Gencell Genética avanzada |
- |
Syn Lab s. f |
|
Uruguay |
South Genetics |
One Light Solution, s.f. |
Longwood s. f Varifarma s. f |
||
|
Paraguay |
South Genetics |
Varifarma |
|||
|
Peru |
Centro Oncológico Aliada GenCode |
Centro Oncológico liada |
Varifarma |
Syn Lab s. f |
|
|
Bolivia |
Instituto BioClínico Cruceño (South Genetics) |
||||
|
Ecuador |
Varifarma |
Syn Lab s. f |
|||
|
CENTRAL AMERICA |
|||||
|
Guatemala |
Integra Cáncer Instituto |
||||
|
Honduras |
Fermilab |
||||
|
CARIBBEAN |
|||||
|
Dominican Republic |
South Genetics |
Medipath Instituto de patología molecular |
|||
|
Puerto Rico |
Puerto Rico Pathology |
Puerto Rico Pathology |
|||
Source: Own elaboration
Table 3
Availability of genetic tests for BrCaH in LAC.
|
Country |
Laboratory |
Test name |
Genetic test specifications |
|
North America |
|||
|
Mexico |
Genetic Services |
Onco Risk |
IAnalysis for 30 genes including BRCA 1 and 2 genes |
|
South Genetics |
myBRCA |
Analysis for BRCA 1 y 2 genes |
|
|
myBRCA HiRisk |
It analyzes 26 genes (including BRCA1 and BRCA2) |
||
|
CentoGen |
BRCA1, BRCA2 Plus |
BRCA1, BRCA2 |
|
|
BRCA1, BRCA2 Combi |
BRCA1, BRCA2 |
||
|
CentoBreast® |
It analyzes 28 genes: including BRCA 1 y 2 |
||
|
CentoCancer |
It analyzes 67 genes: including BRCA 1 y 2 |
||
|
CentoCancer® comprehensive |
Analyzes 118 genes including BRCA 1 y 2 |
||
|
Mayo Clinic |
Not specified |
Análisis de genes BRCA 1/2 |
|
|
Medical Genomics - Molecular Diagnostics Laboratory |
BRCA 1 y 2 (C8838) |
It analyzes BRCA 1/2 genes |
|
|
BRCA Plus (C8836): |
Analysis for 8 genes; including BRCA 1/2 |
||
|
Mexico |
Medical Genomics - Molecular Diagnostics Laboratory |
CancerNext (C8824) |
Analysis 34 genes including los genes BRCA 1/2 |
|
Genos Medica |
BRCA 1/2 (C8838) |
It analyses BRCA ½ genes |
|
|
Milenia Labs |
BRCA 1 y 2 NGS Express |
It analyses BRCA ½ genes |
|
|
Paneles PreSENTIA |
19 panels that analyze specific genes and allow you to target only the type of cancer you want to prevent |
||
|
Patiacan, s. f |
BRCA analysis |
Analysis of genes 1 and 2 BRCA |
|
|
Suramérica |
|||
|
Argentina |
South Genetics |
myBRCA |
Analysis of genes 1 and 2 BRCA |
|
myBRCA HiRisk |
It analyses 26 genes (including BRCA1 y BRCA2) myBRCA y myBRCA HiRisk |
||
|
Centro Nacional de Genética Médica |
Not specified |
Analysis of BRCA 1 and 2 genes |
|
|
IAF Instituto Alexander Fleming, Centro Mamario |
Not specified |
Genetic counseling and genetic testing. |
|
|
LDM Laboratorio de diagnóstico molecular |
Hereditary cancer test |
Not specified |
|
|
Argentina |
ROSSI |
BRCA1 y BRCA2 |
BRCA 1 and 2 Complete Sequencing BRCA1 and BRCA2 - Ashkenazi panel gene mutations BRCA1 & BRCA2 - Ashkenazi - Sephardic panel gene mutations BRCA 1 and BRCA 2 MPLA |
|
Chile |
Clínica Alemana |
Not specified |
Genetic counseling and testing |
|
South Genetics 41 |
Hereditary cancer test |
It analyzes 47 genes including BRCA1 and BRCA2. |
|
|
Biogenetics |
BRCA 1/ 2 |
BRCA 1 y 2 Secuenciación completa |
|
|
Colombia |
South Genetics |
myBRCA |
Analysis of BRCA 1 and 2 genes |
|
myBRCA HiRisk |
It analyzes 26 genes (including BRCA1 and BRCA2). |
||
|
Genetix |
BRACATIX |
Analysis of BRCA 1 and 2 genes |
|
|
BRCATIX PLUS |
Sequencing of 25 genes |
||
|
Oncotix |
It analyzes 51 genes including BRCA1 and BRCA2 |
||
|
Oncopanel |
Analysis of genes of clinical interest according to the patient’s personal and family history. |
||
|
Ecuador |
South Genetics |
myBRCA |
Analysis for BRCA 1 y 2 |
|
myBRCA HiRisk |
It analyses 26 genes (including BRCA1 and BRCA2) |
||
|
SynLab |
BRCA +16 |
It analyses BRCA genes plus 16 no BRCA genes |
|
|
Biocells Genomics |
BreastDetect® |
37 including BRCA1 and BRCA٢ genes |
|
|
Paraguay |
South Genetics |
myBRCA |
Analysis for los genes BRCA 1 y 2 |
|
myBRCA HiRisk |
It analises 26 genes (incluidos los BRCA1 y BRCA2) |
||
|
Biosur |
BRCA 1/2 |
Analysis for los genes BRCA 1 y 2 Muestra requerida: sangre |
|
|
Perú |
South Genetics |
myBRCA |
Analysis for los genes BRCA 1 y 2 |
|
myBRCA HiRisk |
It analyses 26 genes (incluidos los BRCA1 y BRCA2) |
||
|
Syn Lab |
BRCA +16 |
It analises los genes BRCA +16 genes |
|
|
Progenie |
Not specified |
Not specific |
|
|
Uruguay |
South Genetics |
myBRCA |
Analysis for genes BRCA 1 y 2 |
|
myBRCA HiRisk |
It analises 26 genes (incluidos los BRCA1 y BRCA2) |
||
|
ADN Uruguay |
BRCA 1/ 2 |
It analyses los genes BRCA 1/ 2 |
|
|
SENTIS Mama y Ovario |
It analises 26 genes, incluidos BRCA 1/2 |
||
|
Bolivia |
South Genetics |
myBRCA |
It analises los genes BRCA 1/2 |
|
myBRCA HiRisk |
It analises 26 genes (incluidos los BRCA1 y BRCA2) |
||
|
Venezuela |
South Genetics |
myBRCA |
Analysis for los genes BRCA 1 y 2 |
|
myBRCA HiRisk |
It analyses 26 genes; including BRCA1/2 |
||
|
Brazil |
Fleury GENOMICA |
Not specified |
Hereditary breast cancer panel: analysis of 25 genes |
|
Centroamérica |
|||
|
Costa Rica |
Hospital Clínica Bíblica |
BRCA 1/ 2 |
Analysis for BRCA 1/2 |
|
Genetic panel |
Analysis for 83 genes; including BRCA1/2 |
||
|
Hospital Metropolitano |
Not specified |
Blood sampling and shipment to Germany |
|
|
Guatemala |
Integra Cáncer Instituto |
Not specified |
It analyses 61 genes, including BRCA1/. genes It analyses 84 genes; including RCA1/2 |
|
Not specified |
It analises 61 y 84 genes; incluidos BRCA1/2 |
||
|
Honduras |
Fertilabh |
BRCA 1/ 2 |
It analises BRCA 1 / 2 genes |
|
Not specified |
It analyses30 y 32 genes incluidos BRCA 1/2 |
||
|
Panamá |
South Genetics |
myBRCA |
It analises de los genes BRCA 1/ 2 |
|
myBRCA HiRisk |
It analyses 26 genes; including BRCA1/2 |
||
|
Vida tec |
Oncogene |
It analyses 11 genes including BRCA 1/2 |
|
|
MyBRCA |
Analysis for genes BRCA 1/2 |
||
|
myBRCA HiRisk |
Analysis for 26 genes; including BRCA1/2 |
||
|
El Salvador |
Laboratorios Centro ginecológico |
Test BRCA 1 2 |
Analysis for genes BRCA 1/2 |
|
CARIBE |
|||
|
República Dominicana |
South Genetics |
myBRCA |
Analysis for los genes BRCA 1/2 |
|
myBRCA HiRisk |
It analyses 26 genes (including BRCA1 and BRCA2) |
||
|
Medipath Instituto de patología molecular |
BRCA 1/ 2 |
It analises BRCA ½ genes |
|
|
Puerto Rico |
Laboratorio Clínico Principal |
Not specified |
Not specified |
|
Quest Diagnostics. s.f. |
Panel BRCA |
It analises BRCA 1/2 genes |
|
|
BRCA Plus |
It analyses 7 genes; including BRCA1/2 genes |
||
Source: Own elaboration
Works Cited
ADN Uruguay. ONCOLOGIA. AND Uruguay. Consultado el 22 de noviembre del 2023. Disponible en: https://www.adnuruguay.com.uy/oncologia/
Agendia mammaprint. El perfil molecular para definir y vencer su cáncer único. Agendia. Consultado el 13 de diciembre del 2023. Disponible en https://agendia.com/mammaprint/
Agendia. 2023. MammaPrint. Agendia. Consultado el 08 de diciembre del 2023. Disponible en: https://agendia.com/mammaprint/
Aliada Centro oncologico. Cancer de mama. Aliada Centro Oncológico. Accesado el 9 de diciembre del 2023. Disponible en: https://www.aliada.com.pe/cancer/tipos-de-cancer/cancer-de-mama/
Ambios. (s.f.-a). AMBIOS. Consultado el 11 de diciembre del 2023. Disponible en: https://ambiosgroup.com/
American Cancer Society. (20 de septiembre de 2019). Pruebas para la expresión genética del cáncer de seno. American Cancer Society. Consultado el 16 de septiembre del 2023. Disponible en: https://www.cancer.org/es/cancer/cancer-de-seno/comprension-de-un-diagnostico-de-cancer-de-seno/pruebas-para-la-expresion-genetica-del-cancer-de-seno.html
ARGENETICS. (s.f.). Oncología. ARGENETICS. Consultado el 23 de diciembre del 2023. Disponible en https://argeneticsar.com.ar/oncologia
Azim, H, A., Mochiels, S., Zagouri, F., S, Delaloge., Filipits, M., Namer, M., Neven, P., Symmans, W., Thompson, A., André, F., Loi, S., & Swanton, C. (2012). Utility of prognostic genomic tests in breast cancer practice: The IMPAKT 2012 Working Group Consensus Statement. Annals of Oncology, 24:647–654,2013 doi:10.1093/annonc/mds645
Bernet, L., Fernández F., Hardisson D., Chic N., & Pascual, T. (2022). Firmas génicas en el cáncer de mama. Revista de Senología y Patología Mamaria - Journal of Breast Science, Vol. 35. (Núm. S2.) Disponible en: https://www.elsevier.es/es-revista-revista-senologia-patologia-mamaria--131-articulo-firmas-genicas-el-cancer-mama-S0214158222000767
Biocells genomics 2020. Sitio web Biocells genomics. Consultado el 05 de noviembre del 2023. Disponible en https://biocellsgenomics.med.ec/oncologia-cancer-de-mama-y-ovarico/
Biogenetics. Estudio BRCA1 y BRCA2 (susceptibilidad al cáncer de mama hereditario). Biogenetics. Consultado el 12 de diciembre del 2023. Disponible en: https://biogenetics.cl/standard/BRCA.html
BIOSUR. DIAGNOSTICOS GENETICOS Más de 1.000 pruebas genéticas de última generación. BIOSUR. Consultado el 25 de noviembre del 2023. Disponible en: https://www.adn-paraguay.com/standard/diagnostico_enfermedades.html
Brandão, M., Pondé, N., & Piccart-Gebhart, M. (2019). Mammaprint™: a comprehensive review. Future oncology (London, England), 15(2), 207–224. https://doi.org/10.2217/fon-2018-0221
BRCA Panel Plus | Quest Diagnostics. (s.f.). Home | Quest Diagnostics. Consultado el 12 de diciembre del 2023. Disponible en: https://www.questdiagnostics.com/healthcare-professionals/clinical-education-center/faq/faq230#accordion-f6a31a7e79-item-3c06860f04
BREAST | prpathology. (s.f.). prpathology. Consultado el 12 de diciembre del 2023. Disponible en: https://www.prpathology.com/breast
Catherine M. Kelly, Philip S. Bernard, Savitri Krishnamurthy, Bailiang Wang, Mark TW Ebbert, Roy RL Bastien, Kenneth M. Boucher, Elliana Young, Takayuki Iwamoto, Lajos Pusztai. (2012) Acuerdo en la predicción de riesgos entre el ensayo de puntuación de recurrencia de 21 genes (Onco type DX®) y PAM50 Breast Cancer Intrinsic Classifier™ en cáncer de mama con receptor de estrógeno positivo en etapa temprana, The Oncologist , volumen 17, número 4, abril de 2012, páginas 492–498, https://doi.org/ 10.1634/teoncólogo.2012-0007
CentoGen. Portafolio de servicios. CentoGen. Consultado el 31 de octubre del 2023. Disponible en https://www.centoportal.com/order/new/products/analysis-method?queryType=TEST&productCategory=2&diseaseCategory=12
Centro médico ABC. Oncotype. Centro médico ABC. Consultado el 30 de septiembre del 2023. Disponible en: https://centromedicoabc.com/estudios/oncotype/
Centro nacional de genética médica. Enfermedades con diagnóstico molecular disponible. Argentina. Gob. ar. Consultado el 2 de noviembre del 2023. Disponible en: https://www.argentina.gob.ar/salud/anlis/cenagem/censo-nacional-de-recursos-publicos-para-diagnostico-de-enfermedades-geneticas-0
Clínica Alemana. Genética clínica. Clínica Alemana. Consultado el 13 de noviembre del 2023. Disponible en: https://www.clinicaalemana.cl/especialidades/pediatria/subespecialidades/genetica-clinica
DeTroye, A., Gabbett, K., Yi, C., Judice, M., Luu, V., Nelson, B., & Gregory, T. (2022). Genetic testing for patients at risk of hereditary breast and ovarian cancer. JAAPA: Official Journal of the American Academy of Physician Assistants, 35(10), 48–52. https://doi.org/10.1097/01.JAA.0000873796.81961.da
Dowsett, M., Sestak, I., Lopez-Knowles, E., Sidhu, K., Dunbier, A. K., Cowens, J. W., Ferree, S., Storhoff, J., Schaper, C., & Cuzick, J. (2013). Comparison of PAM50 risk of recurrence score with Oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 31(22), 2783–2790. https://doi.org/10.1200/JCO.2012.46.1558
Dubsky, P., Filipits, M., Jakesz, R., Rudas, M., Singer, C. F., Greil, R., Dietze, O., Luisser, I., Klug, E., Sedivy, R., Bachner, M., Mayr, D., Schmidt, M., Gehrmann, M. C., Petry, C., Weber, K. E., Kronenwett, R., Brase, J. C., Gnant, M., & Austrian Breast and Colorectal Cancer Study Group (ABCSG) (2013). EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive and HER2-negative early breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology, 24(3), 640–647. https://doi.org/10.1093/annonc/mds334
Fertilab Centro de diagnóstico y Fertilidad de Honduras. Fertilab. Consultado el 5 de noviembre del 2023. Disponible en https://fertilabh.com/oncologicos
Fleury Genomica. Panel de Cáncer Hereditario de Mama y Ovario con Análisis de CNV. FLeury Genomica. Consultado el 4 de noviembre del 2023. Disponible en: https://www.fleurygenomica.com.br/exames/painel-de-cancer-de-mama -e-ovario-hereditario/?gclid=Cj0KCQiA P6dBhD1ARIsAAGI7HAekL8 LdtAAvazLgX_35_6zP1OH7 EZk012qKmjGIO2QvxAb 5BQEOeUaAubVEALwwcB
Fleury Genomica. Prueba Oncotype DX® para el cáncer de mama invasivo. Fleury Genómica. Consultado el 3 de junio del 2023. Disponible en: https://www.fleurygenomica.com.br/exames/teste-oncotype-dx-para-cancer-de-mama-invasivo/
Gencell Genetica avanzada. Endorpredict. GenCell. Consultado el 4 de noviembre 2023. Disponible en: https://gencellpharma.com/endopredict/
GeneCode S.A.C. (s.f.). GeneCode S.A.C. Consultado el 12 de diciembre del 2023. Disponible en; https://genecode.pe/
Genelab Diagnóstico molecular inteligente. EndoPredict Breast Cancer Recurrence Test. Consultado el 22 de noviembre. Disponible en https://genelab.mx/service/endopredict-breast-cancer-recurrence-test/
Genetic Services. Portafolio de servicios. Genetic Services. Consultado el 25 de septiembre del 2023. Disponible en: https://genethic.mx/servicios
Genetix. Ontogenética. Sitio web de Genetix. Consultado el 10 de noviembre del 2023. Disponible en: https://genetix.com.co/oncogenetica/
Genomic Health, Oncotype DX, Oncotype IQ, Recurrence Score, and Breast Recurrence Score are registered trademarks of Genomic Health, Inc., an Exact Sciences corporation. Consultado el 09 de octubre del 2023. Disponible en: https://www.oncotypeiq.com/es-es/cancer-de-mama/profesional-sanitario/oncotype-dx-breast-recurrence-score/que-es-el-test#
Genómica Medica. Prueba de ADN para cáncer de mama y ovario. Genómica Medica. Consultado el 15 de noviembre del 2023. Disponible es:
Genos médica. Cáncer de mama hereditario. Genos médica. Consultado el 11 de noviembre del 2023. Disponible en: https://www.genosmedica.com/servicios/cm.php
González, L., Bardach, A., Pichon-Riviere, A., Augustovski, F., Martí, S., Alcaraz, A., Ciapponi, A., López, A., & Ares, L. (2015). Biblioteca Virtual en Salud BVS. Pruebas de expresión genómica en pacientes con cáncer de mama: MammaPrint®, OncotypeDX® y Prosigna® / Genomic expression tests in patients with breast cancer: MammaPrint®, Oncotype DX® and Prosigna® Disponible en https://pesquisa.bvsalud.org/portal/resource/es/biblio-986884
Griguolo, G., Bottosso, M., Vernaci, G., Miglietta, F., Dieci, M. V., & Guarneri, V. (2022). Gene-expression signatures to inform neoadjuvant treatment decision in HR+/HER2- breast cancer: Available evidence and clinical implications. Cancer treatment reviews, 102, 102323. https://doi.org/10.1016/j.ctrv.2021.102323
Hospital Clinica BIBLICA. Medicina Genética. Hospital Clínica BIBLICA. Consultado el 28 de noviembre del 2023. Disponible en: https://www.clinicabiblica.com/es/servicios/medicina-genetica
Hospital METROPOLITANO. Centro de cáncer y hematología. Hospital Metropolitano. Consultado el 18 de noviembre del 2023. Disponible en: https://ccm.metropolitanocr.com/
Hospital Vivian Pellas. Laboratorio. Consultado el 01 de diciembre del 2023. Disponible en: https://www.hospitalvivianpellas.com/laboratorio/
IAF Instituto Alexander Fleming. (11 de agosto 2020). Cáncer de mama. IAF. Consultado el 16 de noviembre del 2023. Disponible en: https://alexanderfleming.org/areas-medicas/centro-mamario-alexander-fleming/
Integra Cáncer Instituto. Cáncer de mama: síntomas, diagnóstico y tratamiento. Integra Cancer Instituto. Consultado el 19 de noviembre del 2023. Disponible en: https://integracancerinstitute.com/cancer-de-mama/
Laboratorios Centro ginecológico. Test BRCA 1/2. Consultado el 5 de octubre del 2023. Disponible en https://labocegi.com/producto/test-brca-1-2/
LDM Laboratorio de Diagnóstico Molecular. Detecte el riesgo de cáncer hereditario a través de una prueba genética simple, precisa y no invasiva. LDM. Consultado el 29 de noviembre del 2023. Disponible en:
Linchar, J. A., Venne, V., & Berse, B. (2015). Genetic tests to identify risk for breast cancer. Seminars in oncology nursing, 31(2), 100–107. https://doi.org/10.1016/j.soncn.2015.02.007
Litton, J. K., Burstein, H. J., & Turner, N. C. (2019). Molecular Testing in Breast Cancer. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting, 39, e1–e7. https://doi.org/10.1200/EDBK_237715
LongWood. EndoPredict, LongWood. Consultado el 05 de diciembre del 2023. Disponible en https://www.dlongwood.com/productos/endopredict/
Lulu, Sun., Ariel, Wu., Gregory R. Bean., Ian S. Hagemann., & Chieh-YuLin. (2021) Molecular Testing in Breast Cancer Current Status and Future Directions. J Mol Diagn, 23:1422e1432; https://doi.org/10.1016/j.jmoldx.2021.07.026.
Martínez González, J. de F., & Corriols Molina, M. (2023). Investigación genética del cáncer de mama hereditario en Latinoamérica y el Caribe: una revisión sistemática. Revista Torreón Universitario, 12(35), 125–145. https://doi.org/10.5377/rtu.v12i35.17003
MayoClinic. Prueba para detectar genes BRCA a fin de determinar el riesgo de padecer cáncer de mama y de ovari. MayoClinic. Consultado el 21 de noviembre del 2023. Disponible en: https://www.mayoclinic.org/es-es/tests-procedures/brca-gene-test/about/pac-20384815
Medipath Instituto de patología molecular. Consultado el 26 de octubre del 2023. Disponible en: https://medipath.net/pruebas.cfm
Milenia Labs. Cancer de mama. Sitio Web de Milenia Labs. Consultado el 07 de mayo del 2023. Disponible en: https://milenialabs.com/nuestras-pruebas/cancer/cancer-de-mama/cancer-hereditario
Müller, B. M., Keil, E., Lehmann, A., Winzer, K. J., Richter-Ehrenstein, C., Prinzler, J., Bangemann, N., Reles, A., Stadie, S., Schoenegg, W., Eucker, J., Schmidt, M., Lippek, F., Jöhrens, K., Pahl, S., Sinn, B. V., Budczies, J., Dietel, M., & Denkert, C. (2013). The EndoPredict Gene-Expression Assay in Clinical Practice - Performance and Impact on Clinical Decisions. PloS one, 8(6), e68252. https://doi.org/10.1371/journal.pone.0068252
Myriad Genetics. EndoPredict. Consultado el 11 de noviembre del 2023. Disponible en https://myriadgenetics.eu/endopredict/
NEOGenomics. (2023) Breast Cancer Index (BCI). Neogenomics. Consultado el 29 de noviembre del 2023. Disponible en: https://neogenomics.com/test-menu/breast-cancer-indexr-bci
OMICS Exact sciences. Oncotype Breast Recurrence Score. OMICS. Consultado el 04 de octubre del 2023. Disponible en https://www.oncotypeargentina.com.ar/#contacto
One Light Solution LLC – 2020. MammaPrint. Accesado el 11 de diciembre del 2023. Disponible en: https://www.onelightsolution.com/mammaprint/
Page M y col. 2021. Declaración PRISMA 2020: una guía actualizada para la publicación de revisiones sistemáticas. Rev Esp Cardiol 2021; 74 (9), 790-799. En http://www.prisma-statement.org/documents/Page%20PRISMA%202020%20Spanish.pdf
Patiacan. (s.f.). Patiacan. Consultado el 12 de diciembre del 2023. Disponible en; http://www.patiacan.com.mx/
Productos y Servicios | Grupo Amarey. (s.f.). Grupo Amarey. Accesado el 12 de diciembre del 2023. Disponible en; https://www.grupoamarey.com/productos-y-servicios/
Progenie. Cáncer Hereditario Diagnostico de enfermedades por ADN. Progenie. Consultado el 27 de noviembre del 2023. Disponible en: https://laboratorioprogenie.com/cancer-hereditario/
Prosigna - Synlab. (s.f.). Synlab. Accesado el 12 de diciembre del 2023. Disponible en: https://www.synlab-sd.com/es/exame/prosigna-4/
Prueba de Predisposición Genética – Laboratorio Clínico Principal. (s.f.). Laboratorio Clínico Principal – Laboratorio Clínico y de Referencia. Consultado el 11 de diciembre del 2023. Disponible en: https://labprincipal.com/pruebas-realizadas/prueba-de-presdisposicion-genetica/
Reid, S., Spalluto, L. B., Lang, K., Weidner, A., & Pal, T. (2022). An overview of genetic services delivery for hereditary breast cancer. Breast cancer research and treatment, 191(3), 491–500. https://doi.org/10.1007/s10549-021-06478-z
ROSSI. Estudios médicos. Rossi. Consultado el 12 de noviembre del 2023. Disponible en: https://www.cdrossi.com/estudios-buscador.php?searchinput=BRCA&submit=
Sánchez-Forgach, E., Carpinteyro-Espín, U., Alemán-Áviles, J., & Sánchez-Basurto, C. (2017) Validación y aplicación clínica de MammaPrint® en pacientes con cáncer de mama. Cirugía y Cirujanos. 2017; 85(4) :320-324. Disponible en: https://www.elsevier.es/es-revista-cirugia-cirujanos-139-articulo-validacion-aplicacion-clinica-mammaprint-pacientes-S0009741116301001
Sanft, T., Aktas, B., Schroeder, B., Bossuyt, V., DiGiovanna, M., Abu-Khalaf, M., Chung, G., Silber, A., Hofstatter, E., Mougalian, S., Epstein, L., Hatzis, C., Schnabel, C., & Pusztai, L. (2015). Prospective assessment of the decision-making impact of the Breast Cancer Index in recommending extended adjuvant endocrine therapy for patients with early-stage ER-positive breast cancer. Breast cancer research and treatment, 154(3), 533–541. https://doi.org/10.1007/s10549-015-3631-9
Senocuidado Servicios médicos Mastológicos. PAM50 Prosigna® Firma Genética para el Pronóstico de Cáncer de Mama. 2015. Senocuidado. Consultado el 10 de diciembre del 2023 Disponible en https://www.senocuidado.com.mx/servicios/pam50_laboratorio_genetica_mama_mexico.html
Sestak, I., Martín, M., Dubsky, P., Kronenwett, R., Rojo, F., Cuzick, J., Filipits, M., Ruiz, A., Gradishar, W., Soliman, H., Schwartzberg, L., Buus, R., Hlauschek, D., Rodríguez-Lescure, A., & Gnant, M. (2019). Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine therapy alone. Breast cancer research and treatment, 176(2), 377–386. https://doi.org/10.1007/s10549-019-05226-8
Sgroi, D. C., Sestak, I., Cuzick, J., Zhang, Y., Schnabel, C. A., Schroeder, B., Erlander, M. G., Dunbier, A., Sidhu, K., Lopez-Knowles, E., Goss, P. E., & Dowsett, M. (2013). Prediction of late distant recurrence in patients with estrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. The Lancet. Oncology, 14(11), 1067–1076. https://doi.org/10.1016/S1470-2045(13)70387-5
South Genetic. Test de cáncer de mama y ovario. South Genetics. Consultado el 27 de noviembre del 2023. Disponible en: www.testcancerdemama.com/es/#about
Southgenetics. Oncotype Diagnostics. Southgenetics. Accesado el 6 de septiembre del 2023. Disponible en https://southgenetics.com/oncotype-dx-mama/
SouthGenetics. Prosigna. SouthGenetics. Consultado el 2 de diciembre del 2023. Disponible en: https://www.southgenetics.cl/test-cancer-hereditario/
SYBLAB. Solutions in Diagnostics. BRCA 16+ Prevención del cáncer ginecológico hereditario. SYNLAB. Consultado el 10 de noviembre del 2023. Disponible en: https://www.synlab-sd.com/es/exame/brca-16-4/
Syed Y. Y. (2020). Oncotype DX Breast Recurrence Score®: A Review of its Use in Early-Stage Breast Cancer. Molecular diagnosis & therapy, 24(5), 621–632. Disponible en: https://doi.org/10.1007/s40291-020-00482-7
SYNLAB. BRCA+16 GENES. SYNLAB. Consultado el 14 de noviembre del 2023. Disponible en: https://www.synlab.pe/pruebas_especiales/brca16genes/
Valencia, O. M., Samuel, S. E., Viscusi, R. K., Riall, T. S., Neumayer, L. A., & Aziz, H. (2017). The Role of Genetic Testing in Patients With Breast Cancer: A Review. JAMA surgery, 152(6), 589–594. https://doi.org/10.1001/jamasurg.2017.0552
Vargas-Aguilar, V. M., & Arroyo-Álvarez, K. (2018). Firmas genéticas para cáncer de mama, utilidad clínica y aplicaciones terapéuticas [Gene signatures for breast cancer, clinical utility and therapeutic applications]. Revista médica del Instituto Mexicano del Seguro Social, 56(2), 180–185. Disponible en: https://www.redalyc.org/journal/4577/457754717014/html/
Varifarma. EndoPredict. Varifarma. Accesado el 18 de julio del 2023. Disponible en https://www.varifarma.com/es/productos/endopredict/
VIDATEC Laboratorio clínico Especializado. CÁNCER GÉNETICO. VIDATEC. Consultado el 29 de septiembre del 2023. Disponible en: https://www.vidateclab.com/cancer-genetico/
White, V. B., Walsh, K. K., Foss, K. S., Amacker-North, L., Lenarcic, S., McNeely, L., & White, R. L., Jr (2018). Genetic Testing for Hereditary Breast Cancer: The Decision to Decline. The American Surgeon, 84(1), 154–160. https://pubmed.ncbi.nlm.nih.gov/29428045/
Zhang, Y., Schnabel, C. A., Schroeder, B. E., Jerevall, P. L., Jankowitz, R. C., Fornander, T., Stål, O., Brufsky, A. M., Sgroi, D., & Erlander, M. G. (2013). The breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clinical cancer research: an official journal of the American Association for Cancer Research, 19(15), 4196–4205. https://doi.org/10.1158/1078-0432.CCR-13-0804